Details of the Drug
General Information of Drug (ID: DMFVGXW)
| Drug Name |
AM-1241
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
444912-48-5; AM1241; AM-1241; (2-iodo-5-nitrophenyl)(1-((1-methylpiperidin-2-yl)methyl)-1H-indol-3-yl)methanone; ZUHIXXCLLBMBDW-UHFFFAOYSA-N; AM 1241; GTPL3316; SCHEMBL2030690; CHEMBL408430; CTK8B9184; BDBM21283; MolPort-009-019-655; HMS3651A13; HMS3650F09; MFCD11045986; (R,S)-AM1241; (1-(Methylpiperidin-2-ylmethyl)-3-(2-iodo-5-nitrobenzoyl)indole); ANW-62168; s1544; AKOS016004972; SB19545; CCG-208738; NCGC00165726-04; NCGC00165726-01; HY-18640; TC-150913; LS-192021; KB-206133; AX8233934; SW219858-1; CS-0011638; ST24033932; Z-3231
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
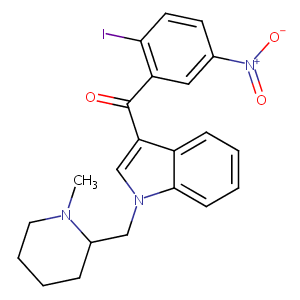 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 503.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
